Details of the Drug
General Information of Drug (ID: DMVW7N3)
| Drug Name |
taurine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
taurine; 2-aminoethanesulfonic acid; 107-35-7; tauphon; L-Taurine; Ethanesulfonic acid, 2-amino-; 2-Aminoethylsulfonic acid; O-Due; 2-Sulfoethylamine; taufon; Aminoethanesulfonic acid; aminoethylsulfonic acid; beta-Aminoethylsulfonic acid; 2-aminoethane-1-sulfonic acid; Taurinum [Latin]; Taurina [Spanish]; Taurine [INN]; FEMA No. 3813; CCRIS 4721; UNII-1EQV5MLY3D; NCI-C60606; AI3-18307; NSC32428; EINECS 203-483-8; NSC 32428; 1EQV5MLY3D; .beta.-Aminoethylsulfonic acid; 1-Aminoethane-2-sulfonic acid; CHEBI:15891
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
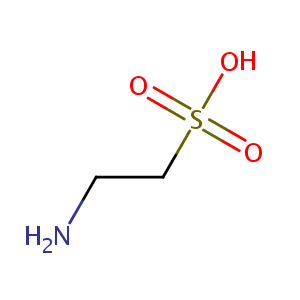 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 125.15 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -4.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
